Make the right device.
Drive market adoption, patient outcomes, and regulatory clearance.
Your program doesn’t have time or money to waste.
Vague directives lead to development rework. Get focused.
clarify customer
& user value
communicate for
stakeholder buy-in
align development with
regulatory standards
translate inputs into
value-dense products
Ryan Held
Principal, Context
With over a decade of MedTech product experience, I’ve guided teams of all size and complexity to successful programs.

product realization services
MedTech specific | software & hardware
-
Prioritize the right problems to solve.
Align your medical technology with customer and user needs.
Validate the business case.
user needs definition
customer (health system) value drivers definition
product architecture definition & prototyping
competitive landscape & differentiation mapping
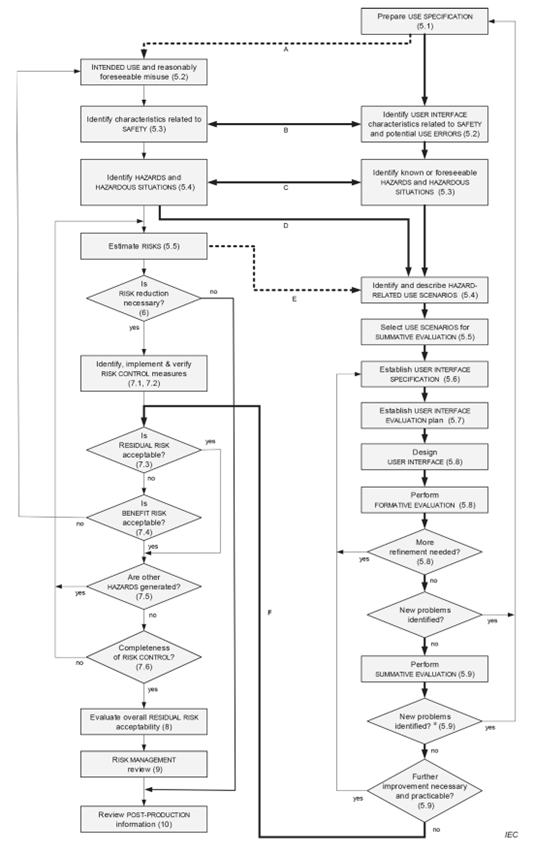
regulatory standards identification (13485, 62366, 60601, 62304, 14971…)
design & usability research
human factors guidance
DHF/MDF initiation
value confirmation testing
-
Get everyone on the same page.
Communicate to investors and sponsors for buy-in.
Align teams for lean development.
development program planning (13485, 21 CFR 820.30)
stakeholder & leadership alignment workshops
investor engagement presentations
product vision & requirements tools
-
Create actionable product requirements.
Accurately translate requirements into pragmatic product design.
Make the right device.
product requirements definition
constraints integration (technology, regulatory, manufacturing)
systems modeling
usability / human factors engineering (62366)
device design (UX, UI, & industrial design)
CAD modeling & prototyping
design engineering (60601)
DHF/MDF documentation

How to get started
Schedule a call
2. Review your proposal
3. Kick-off your project
Contact Us
Interested in working together? Fill out some info and we will be in touch shortly. Can’t wait to hear from you!